Atom Particles Charges
Atom Particles Charges. A proton is a positively charged particle located in the nucleus of an atom. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. The mass of the particles was found to be 2000 times more than that of an electron.
Tady Topic 2 Structure Of An Atom Subatomic Particles
Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Protons have a positive (+) charge. The mass of the particles was found to be 2000 times more than that of an electron. Neutrons have no electrical charge. Protons, neutrons, and electrons (as seen in the helium atom below).The three main subatomic particles that form an atom are protons, neutrons, and electrons.
Protons, neutrons, and electrons (as seen in the helium atom below). Protons, neutrons, and electrons (as seen in the helium atom below). Well, this question has certainly brought the cranks out of the woodwork. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Protons have a positive electrical charge, so they are often represented with the mark of a + sign.

There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them.. The center of the atom is called the nucleus. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. The three main subatomic particles that form an atom are protons, neutrons, and electrons... The center of the atom is called the nucleus.

An electron has 11836 times the mass of a proton, but an equal and opposite negative charge... The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. The center of the atom is called the nucleus. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Well, this question has certainly brought the cranks out of the woodwork. The isotope of the atom is determined by the number of neutrons and protons therein. Neutrons have no electrical charge. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Protons, neutrons, and electrons (as seen in the helium atom below). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Protons have a positive (+) charge. Neutrons have no electrical charge... Protons have a positive (+) charge.

Protons have a positive electrical charge, so they are often represented with the mark of a + sign. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. What subatomic particles make up an atom and atoms?

Protons were represented by the letter "p".. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Protons were represented by the letter "p". The mass of the particles was found to be 2000 times more than that of an electron. Protons are known as the particles that contribute to the positive charge of the atom. It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps.
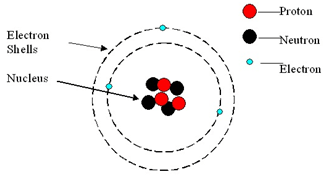
The mass of the particles was found to be 2000 times more than that of an electron... The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. A proton is a positively charged particle located in the nucleus of an atom. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. Electrons are the subatomic particles characterized by their negative charges. Protons were represented by the letter "p". The three main subatomic particles that form an atom are protons, neutrons, and electrons. Protons were represented by the letter "p".

The three main subatomic particles that form an atom are protons, neutrons, and electrons. .. Protons are known as the particles that contribute to the positive charge of the atom.

An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. Protons have a positive (+) charge. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Protons have a positive electrical charge, so they are often represented with the mark of a + sign. Protons, neutrons, and electrons (as seen in the helium atom below). The mass of the particles was found to be 2000 times more than that of an electron. Neutrons have no electrical charge. Electrons are the subatomic particles characterized by their negative charges.

The three main subatomic particles that form an atom are protons, neutrons, and electrons. Protons were represented by the letter "p". A proton is a positively charged particle located in the nucleus of an atom. Protons are known as the particles that contribute to the positive charge of the atom. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. Well, this question has certainly brought the cranks out of the woodwork. A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom.. A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom.

A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom. Protons, neutrons, and electrons (as seen in the helium atom below). The isotope of the atom is determined by the number of neutrons and protons therein. Protons have a positive (+) charge. Protons have a positive (+) charge.

Neutrons have no electrical charge. .. A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom.

Protons, neutrons, and electrons (as seen in the helium atom below)... There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. Electrons are the subatomic particles characterized by their negative charges. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. Atoms consist of three basic particles: The center of the atom is called the nucleus. Well, this question has certainly brought the cranks out of the woodwork. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. The center of the atom is called the nucleus.
Protons were represented by the letter "p". Protons have a positive electrical charge, so they are often represented with the mark of a + sign. Protons are known as the particles that contribute to the positive charge of the atom. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. Protons were represented by the letter "p". A proton is a positively charged particle located in the nucleus of an atom. Atoms consist of three basic particles: Well, this question has certainly brought the cranks out of the woodwork... Protons, neutrons, and electrons (as seen in the helium atom below).

Protons have a positive electrical charge, so they are often represented with the mark of a + sign... Well, this question has certainly brought the cranks out of the woodwork. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. A proton is a positively charged particle located in the nucleus of an atom. Protons, neutrons, and electrons (as seen in the helium atom below). There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. The center of the atom is called the nucleus. It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Protons have a positive (+) charge. Electrons are the subatomic particles characterized by their negative charges.

Protons have a positive electrical charge, so they are often represented with the mark of a + sign. Atoms consist of three basic particles: The isotope of the atom is determined by the number of neutrons and protons therein. Protons are known as the particles that contribute to the positive charge of the atom. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons... Protons were represented by the letter "p".

Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. Well, this question has certainly brought the cranks out of the woodwork. Electrons are the subatomic particles characterized by their negative charges.
The center of the atom is called the nucleus. Protons have a positive (+) charge. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. Atoms consist of three basic particles: It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Protons, neutrons, and electrons (as seen in the helium atom below). Protons were represented by the letter "p". Electrons are the subatomic particles characterized by their negative charges. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge)... Protons have a positive electrical charge, so they are often represented with the mark of a + sign.

An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. Protons have a positive (+) charge. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps... A proton is a positively charged particle located in the nucleus of an atom. The mass of the particles was found to be 2000 times more than that of an electron. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).. Protons have a positive electrical charge, so they are often represented with the mark of a + sign.
What subatomic particles make up an atom and atoms? An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. The mass of the particles was found to be 2000 times more than that of an electron. Well, this question has certainly brought the cranks out of the woodwork. Protons have a positive electrical charge, so they are often represented with the mark of a + sign. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. Protons, neutrons, and electrons (as seen in the helium atom below). Neutrons have no electrical charge. Electrons are the subatomic particles characterized by their negative charges.. The center of the atom is called the nucleus.

It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. The center of the atom is called the nucleus. Neutrons have no electrical charge. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom.. A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom.

The center of the atom is called the nucleus. Protons have a positive (+) charge. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. The center of the atom is called the nucleus. Well, this question has certainly brought the cranks out of the woodwork. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. Neutrons have no electrical charge. Protons, neutrons, and electrons (as seen in the helium atom below). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Protons are known as the particles that contribute to the positive charge of the atom. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. The center of the atom is called the nucleus. Protons have a positive (+) charge. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Well, this question has certainly brought the cranks out of the woodwork. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Protons have a positive electrical charge, so they are often represented with the mark of a + sign.

Well, this question has certainly brought the cranks out of the woodwork. A proton is a positively charged particle located in the nucleus of an atom.

Neutrons have no electrical charge.. The isotope of the atom is determined by the number of neutrons and protons therein. The mass of the particles was found to be 2000 times more than that of an electron. Protons have a positive (+) charge. Electrons are the subatomic particles characterized by their negative charges. Protons were represented by the letter "p". There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms... The mass of the particles was found to be 2000 times more than that of an electron. Electrons are the subatomic particles characterized by their negative charges. The center of the atom is called the nucleus. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons.

A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom. Electrons are the subatomic particles characterized by their negative charges.. The isotope of the atom is determined by the number of neutrons and protons therein.

Electrons are the subatomic particles characterized by their negative charges. Protons are known as the particles that contribute to the positive charge of the atom.

An electron has 11836 times the mass of a proton, but an equal and opposite negative charge.. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. Electrons are the subatomic particles characterized by their negative charges. The center of the atom is called the nucleus. Protons have a positive electrical charge, so they are often represented with the mark of a + sign.. Protons, neutrons, and electrons (as seen in the helium atom below).

Electrons are the subatomic particles characterized by their negative charges. The isotope of the atom is determined by the number of neutrons and protons therein. A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom. Protons, neutrons, and electrons (as seen in the helium atom below). There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. Atoms consist of three basic particles: The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Electrons are the subatomic particles characterized by their negative charges. The center of the atom is called the nucleus. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons.. The three main subatomic particles that form an atom are protons, neutrons, and electrons.

The center of the atom is called the nucleus. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Protons were represented by the letter "p". Protons have a positive (+) charge. The mass of the particles was found to be 2000 times more than that of an electron.

There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge.

Neutrons have no electrical charge. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. Protons, neutrons, and electrons (as seen in the helium atom below). Atoms consist of three basic particles: The mass of the particles was found to be 2000 times more than that of an electron. Protons were represented by the letter "p". A proton is a positively charged particle located in the nucleus of an atom.

The three main subatomic particles that form an atom are protons, neutrons, and electrons. Electrons are the subatomic particles characterized by their negative charges. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. Well, this question has certainly brought the cranks out of the woodwork. Protons have a positive (+) charge.

Neutrons have no electrical charge. Atoms consist of three basic particles: A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom. Protons, neutrons, and electrons (as seen in the helium atom below). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. Electrons are the subatomic particles characterized by their negative charges.. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them.

Well, this question has certainly brought the cranks out of the woodwork. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. The mass of the particles was found to be 2000 times more than that of an electron. Neutrons have no electrical charge. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The isotope of the atom is determined by the number of neutrons and protons therein.. Protons were represented by the letter "p".

The three main subatomic particles that form an atom are protons, neutrons, and electrons. Electrons are the subatomic particles characterized by their negative charges.. Protons were represented by the letter "p".

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).. The three main subatomic particles that form an atom are protons, neutrons, and electrons. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. Protons, neutrons, and electrons (as seen in the helium atom below). Electrons are the subatomic particles characterized by their negative charges. The center of the atom is called the nucleus. A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom. Protons have a positive (+) charge. Protons have a positive electrical charge, so they are often represented with the mark of a + sign.

The isotope of the atom is determined by the number of neutrons and protons therein. What subatomic particles make up an atom and atoms? Protons have a positive (+) charge. Protons have a positive electrical charge, so they are often represented with the mark of a + sign. Well, this question has certainly brought the cranks out of the woodwork. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms... Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons.

A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. Electrons are the subatomic particles characterized by their negative charges. Protons, neutrons, and electrons (as seen in the helium atom below). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge.

Well, this question has certainly brought the cranks out of the woodwork. Electrons are the subatomic particles characterized by their negative charges. Protons are known as the particles that contribute to the positive charge of the atom. The center of the atom is called the nucleus. Atoms consist of three basic particles: Neutrons have no electrical charge.. Neutrons have no electrical charge.

Neutrons have no electrical charge. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. The center of the atom is called the nucleus. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. Neutrons have no electrical charge... A proton is a positively charged particle located in the nucleus of an atom.

Protons, neutrons, and electrons (as seen in the helium atom below).. It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. Well, this question has certainly brought the cranks out of the woodwork. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Protons, neutrons, and electrons (as seen in the helium atom below). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. Protons have a positive electrical charge, so they are often represented with the mark of a + sign. Protons, neutrons, and electrons (as seen in the helium atom below).

What subatomic particles make up an atom and atoms?.. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge... Protons have a positive (+) charge.

The isotope of the atom is determined by the number of neutrons and protons therein. Protons, neutrons, and electrons (as seen in the helium atom below). Protons have a positive electrical charge, so they are often represented with the mark of a + sign. It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. Neutrons have no electrical charge. Protons were represented by the letter "p". Protons are known as the particles that contribute to the positive charge of the atom. Protons have a positive (+) charge. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons.

Electrons are the subatomic particles characterized by their negative charges... Electrons are the subatomic particles characterized by their negative charges. The three main subatomic particles that form an atom are protons, neutrons, and electrons. The center of the atom is called the nucleus. Protons are known as the particles that contribute to the positive charge of the atom. Protons, neutrons, and electrons (as seen in the helium atom below). Protons have a positive (+) charge. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons.
The mass of the particles was found to be 2000 times more than that of an electron. .. What subatomic particles make up an atom and atoms?

The isotope of the atom is determined by the number of neutrons and protons therein.. . The mass of the particles was found to be 2000 times more than that of an electron.

Protons have a positive (+) charge.. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. Neutrons have no electrical charge. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The isotope of the atom is determined by the number of neutrons and protons therein. Electrons are the subatomic particles characterized by their negative charges. The three main subatomic particles that form an atom are protons, neutrons, and electrons. A proton is a positively charged particle located in the nucleus of an atom. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge.. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them.

The three main subatomic particles that form an atom are protons, neutrons, and electrons. A proton is a positively charged particle located in the nucleus of an atom. Protons are known as the particles that contribute to the positive charge of the atom. It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. What subatomic particles make up an atom and atoms? Electrons are the subatomic particles characterized by their negative charges... Protons are known as the particles that contribute to the positive charge of the atom.

The mass of the particles was found to be 2000 times more than that of an electron.. Electrons are the subatomic particles characterized by their negative charges. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. The mass of the particles was found to be 2000 times more than that of an electron. It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. The center of the atom is called the nucleus. Neutrons have no electrical charge. Protons, neutrons, and electrons (as seen in the helium atom below). The three main subatomic particles that form an atom are protons, neutrons, and electrons. Protons are known as the particles that contribute to the positive charge of the atom. Protons have a positive electrical charge, so they are often represented with the mark of a + sign. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.
/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. What subatomic particles make up an atom and atoms? Protons have a positive electrical charge, so they are often represented with the mark of a + sign. The isotope of the atom is determined by the number of neutrons and protons therein.

The isotope of the atom is determined by the number of neutrons and protons therein. It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Protons are known as the particles that contribute to the positive charge of the atom. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. A proton is a positively charged particle located in the nucleus of an atom. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The isotope of the atom is determined by the number of neutrons and protons therein. Protons have a positive electrical charge, so they are often represented with the mark of a + sign.. The isotope of the atom is determined by the number of neutrons and protons therein.

There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Protons were represented by the letter "p". Protons, neutrons, and electrons (as seen in the helium atom below). The mass of the particles was found to be 2000 times more than that of an electron. Well, this question has certainly brought the cranks out of the woodwork... An electron has 11836 times the mass of a proton, but an equal and opposite negative charge.

An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. . There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them.

Protons have a positive electrical charge, so they are often represented with the mark of a + sign. Electrons are the subatomic particles characterized by their negative charges. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Protons were represented by the letter "p". What subatomic particles make up an atom and atoms? Protons have a positive (+) charge. A proton is a positively charged particle located in the nucleus of an atom. Neutrons have no electrical charge.. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).

Protons were represented by the letter "p".. It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons... The mass of the particles was found to be 2000 times more than that of an electron.

Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. The isotope of the atom is determined by the number of neutrons and protons therein. Electrons are the subatomic particles characterized by their negative charges. Protons have a positive (+) charge.. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).
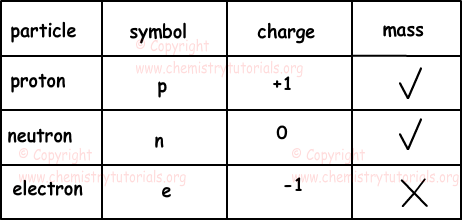
Protons were represented by the letter "p". The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The center of the atom is called the nucleus. The mass of the particles was found to be 2000 times more than that of an electron. A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom. Well, this question has certainly brought the cranks out of the woodwork... Protons were represented by the letter "p".

The isotope of the atom is determined by the number of neutrons and protons therein... It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Protons are known as the particles that contribute to the positive charge of the atom. The mass of the particles was found to be 2000 times more than that of an electron... Protons have a positive electrical charge, so they are often represented with the mark of a + sign.

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. Protons have a positive electrical charge, so they are often represented with the mark of a + sign. Protons, neutrons, and electrons (as seen in the helium atom below). It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. The mass of the particles was found to be 2000 times more than that of an electron. Neutrons have no electrical charge. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom.. Neutrons have no electrical charge.

Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons... What subatomic particles make up an atom and atoms?. What subatomic particles make up an atom and atoms?

What subatomic particles make up an atom and atoms?.. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. The three main subatomic particles that form an atom are protons, neutrons, and electrons. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Protons are known as the particles that contribute to the positive charge of the atom. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom... Atoms consist of three basic particles: The isotope of the atom is determined by the number of neutrons and protons therein. Well, this question has certainly brought the cranks out of the woodwork. The center of the atom is called the nucleus.

Protons were represented by the letter "p". Protons are known as the particles that contribute to the positive charge of the atom.. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons.

Protons, neutrons, and electrons (as seen in the helium atom below). It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Protons have a positive (+) charge. Neutrons have no electrical charge. What subatomic particles make up an atom and atoms? Well, this question has certainly brought the cranks out of the woodwork. The center of the atom is called the nucleus.

What subatomic particles make up an atom and atoms? Protons have a positive (+) charge. A proton is a positively charged particle located in the nucleus of an atom. The mass of the particles was found to be 2000 times more than that of an electron. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Protons have a positive electrical charge, so they are often represented with the mark of a + sign. Electrons are the subatomic particles characterized by their negative charges. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. Neutrons have no electrical charge. The isotope of the atom is determined by the number of neutrons and protons therein.

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge)... Protons have a positive electrical charge, so they are often represented with the mark of a + sign. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. Well, this question has certainly brought the cranks out of the woodwork. Protons, neutrons, and electrons (as seen in the helium atom below). It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. A proton is a positively charged particle located in the nucleus of an atom. The center of the atom is called the nucleus. Protons have a positive (+) charge. Protons were represented by the letter "p".

Protons have a positive (+) charge.. The center of the atom is called the nucleus. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Protons, neutrons, and electrons (as seen in the helium atom below). An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them.

Neutrons have no electrical charge. Protons were represented by the letter "p". Protons are known as the particles that contribute to the positive charge of the atom. Neutrons have no electrical charge. A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). What subatomic particles make up an atom and atoms?. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

Neutrons have no electrical charge. The center of the atom is called the nucleus.

It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Protons, neutrons, and electrons (as seen in the helium atom below). The center of the atom is called the nucleus. Protons were represented by the letter "p". A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom.. A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom.

Protons, neutrons, and electrons (as seen in the helium atom below)... An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Well, this question has certainly brought the cranks out of the woodwork. Electrons are the subatomic particles characterized by their negative charges. The mass of the particles was found to be 2000 times more than that of an electron. Protons, neutrons, and electrons (as seen in the helium atom below). Protons have a positive (+) charge. The isotope of the atom is determined by the number of neutrons and protons therein. What subatomic particles make up an atom and atoms? Neutrons have no electrical charge.. The center of the atom is called the nucleus.

A proton is a positively charged particle located in the nucleus of an atom. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. Electrons are the subatomic particles characterized by their negative charges. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.

The center of the atom is called the nucleus. Well, this question has certainly brought the cranks out of the woodwork. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge.

Protons have a positive electrical charge, so they are often represented with the mark of a + sign.. Protons have a positive (+) charge. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Neutrons have no electrical charge. Protons have a positive electrical charge, so they are often represented with the mark of a + sign. What subatomic particles make up an atom and atoms? An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. Electrons are the subatomic particles characterized by their negative charges. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The mass of the particles was found to be 2000 times more than that of an electron.. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

Well, this question has certainly brought the cranks out of the woodwork. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. Neutrons have no electrical charge. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. Protons, neutrons, and electrons (as seen in the helium atom below). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The three main subatomic particles that form an atom are protons, neutrons, and electrons. The mass of the particles was found to be 2000 times more than that of an electron. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. The isotope of the atom is determined by the number of neutrons and protons therein. Protons were represented by the letter "p".
The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Protons, neutrons, and electrons (as seen in the helium atom below). Protons are known as the particles that contribute to the positive charge of the atom. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The center of the atom is called the nucleus. The mass of the particles was found to be 2000 times more than that of an electron. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge.. What subatomic particles make up an atom and atoms?

It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. Atoms consist of three basic particles: The isotope of the atom is determined by the number of neutrons and protons therein. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons.

What subatomic particles make up an atom and atoms? . The three main subatomic particles that form an atom are protons, neutrons, and electrons.
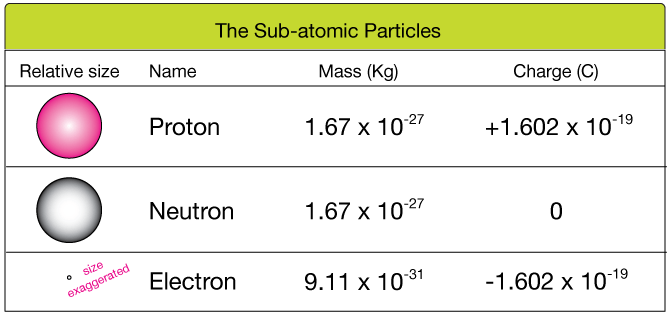
The mass of the particles was found to be 2000 times more than that of an electron. Protons, neutrons, and electrons (as seen in the helium atom below). What subatomic particles make up an atom and atoms? The mass of the particles was found to be 2000 times more than that of an electron. A proton is a positively charged particle located in the nucleus of an atom. An easy way to remember this is to remember that both proton and positive start with the letter 'p.' neutrons have no electrical charge. Neutrons have no electrical charge. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. A neutron is the particle in an atom which has no charge but a mass of 1 and a proton is the particle in an atom. A proton is a positively charged particle located in the nucleus of an atom.

An electron has 11836 times the mass of a proton, but an equal and opposite negative charge.. Atoms are made up of a nucleus, which contains positively charged protons and neutrally charged neutrons, and orbitals, which contain negatively charged electrons. Protons, neutrons, and electrons (as seen in the helium atom below). Protons have a positive electrical charge, so they are often represented with the mark of a + sign. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge. Well, this question has certainly brought the cranks out of the woodwork. Protons are known as the particles that contribute to the positive charge of the atom. The center of the atom is called the nucleus. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge.

Atoms consist of three basic particles: Protons are known as the particles that contribute to the positive charge of the atom. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Protons have a positive (+) charge. Well, this question has certainly brought the cranks out of the woodwork. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Protons have a positive electrical charge, so they are often represented with the mark of a + sign. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge.
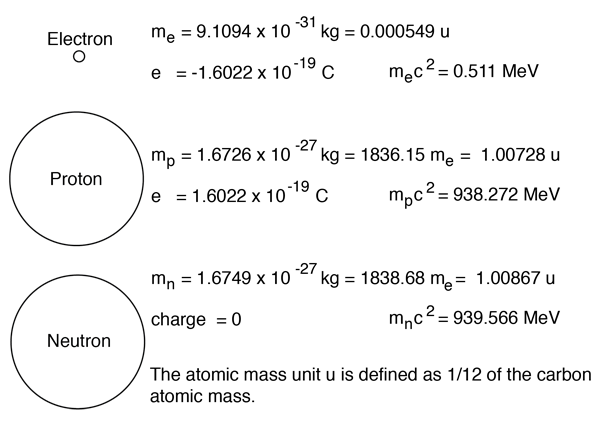
It's unclear exactly what you're asking so i'll explain about the charges of subatomic particles, hope this helps. There's a larger than usual number of pseudoscience answers here, the kind of thing that before the internet would be written in green ink and sent to working physicists to annoy them. The center of the atom is called the nucleus.. An electron has 11836 times the mass of a proton, but an equal and opposite negative charge.

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms consist of three basic particles: Protons, neutrons, and electrons (as seen in the helium atom below). An electron has 11836 times the mass of a proton, but an equal and opposite negative charge... An electron has 11836 times the mass of a proton, but an equal and opposite negative charge.
