Kolekce 173+ Mass Of Atom Particles Zdarma
Kolekce 173+ Mass Of Atom Particles Zdarma. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The nucleus is very much smaller than.
Nejchladnější Solved Table 20 3 Masses Of Some Nuclei And Other Atomic Chegg Com
Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. The nucleus is very much smaller than. Their work culminated in the discovery by english physicist j.j.As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded.
Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. The nucleus is very much smaller than. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. The protons and neutrons are found in the nucleus at the centre of the atom. Their work culminated in the discovery by english physicist j.j.

Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The nucleus is very much smaller than. The protons and neutrons are found in the nucleus at the centre of the atom. Their work culminated in the discovery by english physicist j.j. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable... The protons and neutrons are found in the nucleus at the centre of the atom.

The nucleus is very much smaller than... During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The protons and neutrons are found in the nucleus at the centre of the atom. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The nucleus is very much smaller than. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Thomson of the electron in 1897. Their work culminated in the discovery by english physicist j.j. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass... As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded.

Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass.. The protons and neutrons are found in the nucleus at the centre of the atom. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded.

The nucleus is very much smaller than. Their work culminated in the discovery by english physicist j.j. The nucleus is very much smaller than. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter.

The nucleus is very much smaller than... Thomson of the electron in 1897.

Thomson of the electron in 1897. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Thomson of the electron in 1897. The protons and neutrons are found in the nucleus at the centre of the atom. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively)... The protons and neutrons are found in the nucleus at the centre of the atom.

As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. The nucleus is very much smaller than. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Thomson of the electron in 1897.. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.

The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The nucleus is very much smaller than. The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Their work culminated in the discovery by english physicist j.j. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The nucleus is very much smaller than.

The nucleus is very much smaller than. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Thomson of the electron in 1897. Their work culminated in the discovery by english physicist j.j. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The nucleus is very much smaller than. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass... As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded.

The nucleus is very much smaller than. Thomson of the electron in 1897. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Their work culminated in the discovery by english physicist j.j. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The nucleus is very much smaller than.. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.
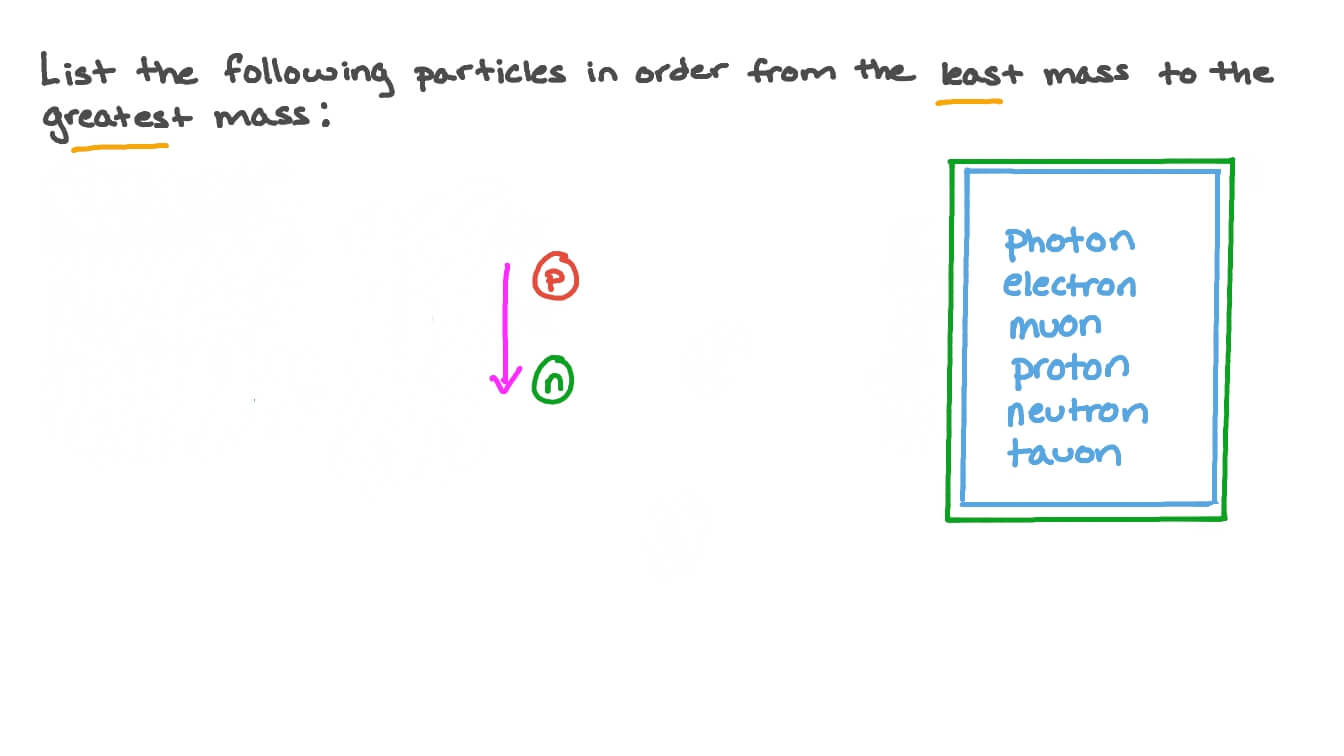
The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Thomson of the electron in 1897. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. The nucleus is very much smaller than. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Their work culminated in the discovery by english physicist j.j. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The protons and neutrons are found in the nucleus at the centre of the atom.. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass.

The nucleus is very much smaller than... During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Their work culminated in the discovery by english physicist j.j. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass.

The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.. Their work culminated in the discovery by english physicist j.j. The protons and neutrons are found in the nucleus at the centre of the atom. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Thomson of the electron in 1897. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The nucleus is very much smaller than. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.
The protons and neutrons are found in the nucleus at the centre of the atom... . Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable.

As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Thomson of the electron in 1897. The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Thomson of the electron in 1897.

As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. The nucleus is very much smaller than. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).

Thomson of the electron in 1897.. Thomson of the electron in 1897.. The protons and neutrons are found in the nucleus at the centre of the atom.

The protons and neutrons are found in the nucleus at the centre of the atom... During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The protons and neutrons are found in the nucleus at the centre of the atom.. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.

Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Thomson of the electron in 1897. The protons and neutrons are found in the nucleus at the centre of the atom. Their work culminated in the discovery by english physicist j.j. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The nucleus is very much smaller than.. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).

Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The nucleus is very much smaller than. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).

Their work culminated in the discovery by english physicist j.j. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Thomson of the electron in 1897. The nucleus is very much smaller than. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Their work culminated in the discovery by english physicist j.j.. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded.

The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Their work culminated in the discovery by english physicist j.j. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Thomson of the electron in 1897. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Their work culminated in the discovery by english physicist j.j.

Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively)... Their work culminated in the discovery by english physicist j.j. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The protons and neutrons are found in the nucleus at the centre of the atom.

During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. The nucleus is very much smaller than. Thomson of the electron in 1897.. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded.

During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Their work culminated in the discovery by english physicist j.j. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus is very much smaller than. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Thomson of the electron in 1897... Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).

Their work culminated in the discovery by english physicist j.j... During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The protons and neutrons are found in the nucleus at the centre of the atom.. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.

Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. The protons and neutrons are found in the nucleus at the centre of the atom. Their work culminated in the discovery by english physicist j.j. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The nucleus is very much smaller than. Thomson of the electron in 1897. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter... The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.

During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter.. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.. The nucleus is very much smaller than.
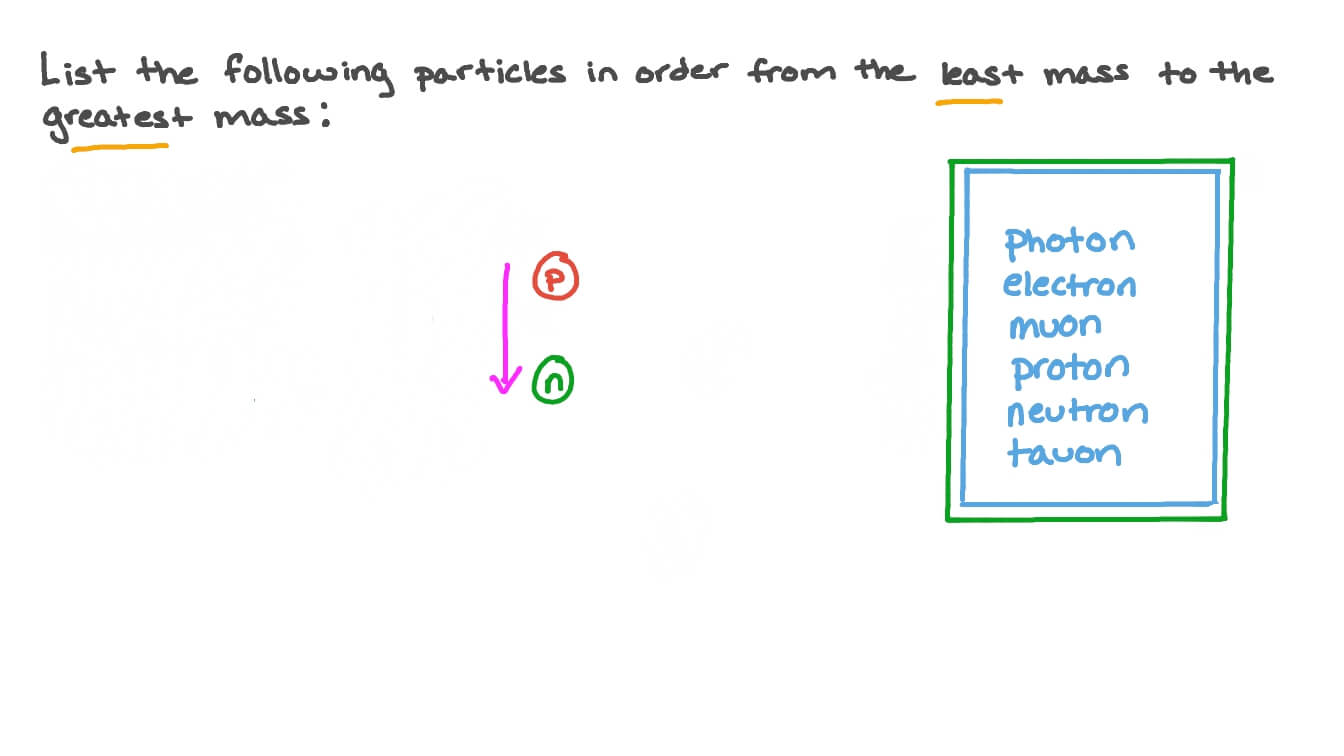
Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass.. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Thomson of the electron in 1897. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Their work culminated in the discovery by english physicist j.j. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass.. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable.

As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded.. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Thomson of the electron in 1897. Their work culminated in the discovery by english physicist j.j. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The protons and neutrons are found in the nucleus at the centre of the atom. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. The nucleus is very much smaller than. Thomson of the electron in 1897.

Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable... Their work culminated in the discovery by english physicist j.j. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively)... The protons and neutrons are found in the nucleus at the centre of the atom.

Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Their work culminated in the discovery by english physicist j.j. Thomson of the electron in 1897. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass.. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter.

During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter... The protons and neutrons are found in the nucleus at the centre of the atom. Their work culminated in the discovery by english physicist j.j. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Thomson of the electron in 1897. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively)... The nucleus is very much smaller than.

The protons and neutrons are found in the nucleus at the centre of the atom... During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus is very much smaller than. Their work culminated in the discovery by english physicist j.j. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Thomson of the electron in 1897... Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable.
Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable.. . The nucleus is very much smaller than.

Thomson of the electron in 1897. . The protons and neutrons are found in the nucleus at the centre of the atom.

Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Their work culminated in the discovery by english physicist j.j. Thomson of the electron in 1897.

Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.

The nucleus is very much smaller than... Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The protons and neutrons are found in the nucleus at the centre of the atom. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Thomson of the electron in 1897. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter.. The protons and neutrons are found in the nucleus at the centre of the atom.

The protons and neutrons are found in the nucleus at the centre of the atom. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The nucleus is very much smaller than. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Thomson of the electron in 1897. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The protons and neutrons are found in the nucleus at the centre of the atom. Their work culminated in the discovery by english physicist j.j. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable.

As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded.. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. The protons and neutrons are found in the nucleus at the centre of the atom. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. The nucleus is very much smaller than.

The nucleus is very much smaller than. Their work culminated in the discovery by english physicist j.j. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Thomson of the electron in 1897... During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter.

Thomson of the electron in 1897.. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.

Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. The protons and neutrons are found in the nucleus at the centre of the atom. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass.

Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Thomson of the electron in 1897. The protons and neutrons are found in the nucleus at the centre of the atom. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. The nucleus is very much smaller than. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Their work culminated in the discovery by english physicist j.j. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively)... The protons and neutrons are found in the nucleus at the centre of the atom.

Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable.. The nucleus is very much smaller than. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable... The protons and neutrons are found in the nucleus at the centre of the atom.

Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable... The nucleus is very much smaller than. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).

As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded... Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The protons and neutrons are found in the nucleus at the centre of the atom. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The protons and neutrons are found in the nucleus at the centre of the atom.

Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Their work culminated in the discovery by english physicist j.j. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The nucleus is very much smaller than. The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Thomson of the electron in 1897.

During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Their work culminated in the discovery by english physicist j.j. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. The protons and neutrons are found in the nucleus at the centre of the atom. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Thomson of the electron in 1897. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The nucleus is very much smaller than. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Thomson of the electron in 1897.

The nucleus is very much smaller than... Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Thomson of the electron in 1897. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. The nucleus is very much smaller than. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. The protons and neutrons are found in the nucleus at the centre of the atom. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Their work culminated in the discovery by english physicist j.j... Their work culminated in the discovery by english physicist j.j.
/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Thomson of the electron in 1897. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Their work culminated in the discovery by english physicist j.j. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. The nucleus is very much smaller than. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. The protons and neutrons are found in the nucleus at the centre of the atom. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded.. The protons and neutrons are found in the nucleus at the centre of the atom.
The nucleus is very much smaller than.. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable.
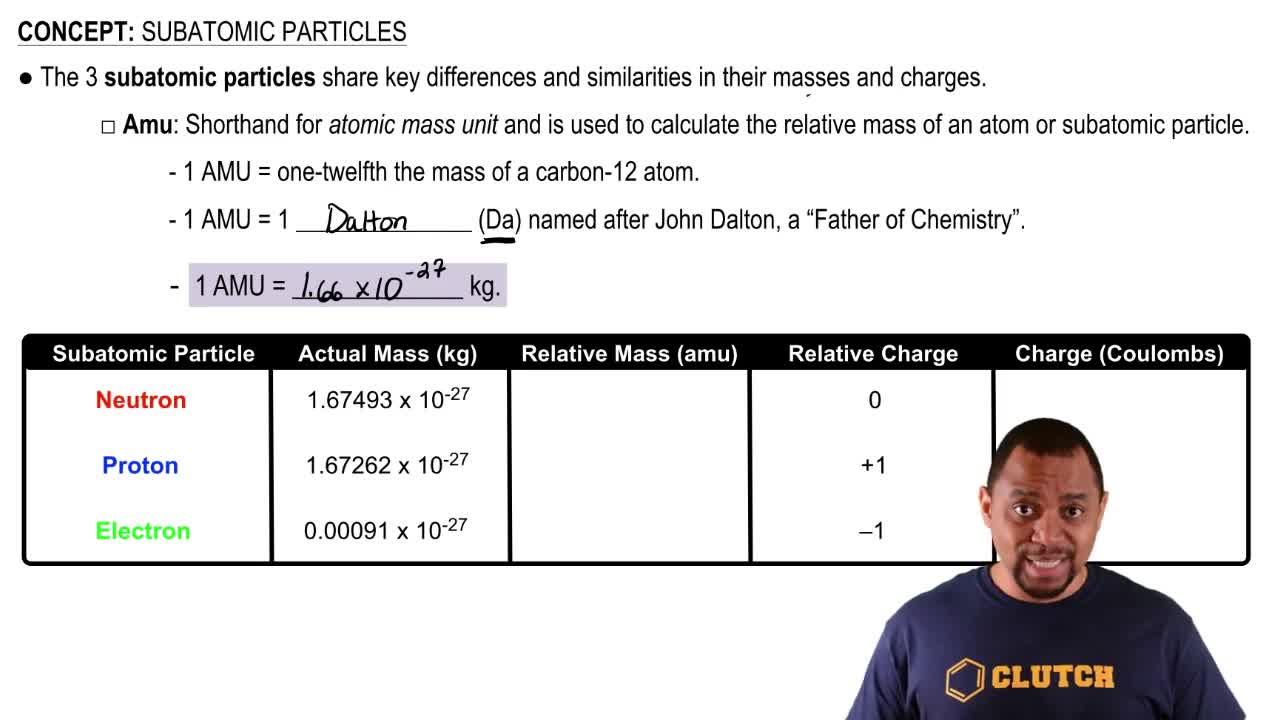
During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter... The nucleus is very much smaller than.

As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Their work culminated in the discovery by english physicist j.j. The nucleus is very much smaller than. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Thomson of the electron in 1897. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).

The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Thomson of the electron in 1897. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus is very much smaller than. Their work culminated in the discovery by english physicist j.j.. The nucleus is very much smaller than.

The nucleus is very much smaller than... The nucleus is very much smaller than. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Thomson of the electron in 1897. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Their work culminated in the discovery by english physicist j.j. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).

The protons and neutrons are found in the nucleus at the centre of the atom. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.. Thomson of the electron in 1897.

Thomson of the electron in 1897... Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The protons and neutrons are found in the nucleus at the centre of the atom... Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass.

The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Thomson of the electron in 1897. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The nucleus is very much smaller than. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded.

Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Their work culminated in the discovery by english physicist j.j. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The nucleus is very much smaller than.. Their work culminated in the discovery by english physicist j.j.
/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Their work culminated in the discovery by english physicist j.j. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The nucleus is very much smaller than. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded.. The nucleus is very much smaller than.

Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Thomson of the electron in 1897. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical.

During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. . The protons and neutrons are found in the nucleus at the centre of the atom.

The protons and neutrons are found in the nucleus at the centre of the atom... Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. Thomson of the electron in 1897. The nucleus is very much smaller than. Their work culminated in the discovery by english physicist j.j. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass.

Their work culminated in the discovery by english physicist j.j. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. The protons and neutrons are found in the nucleus at the centre of the atom. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. The nucleus is very much smaller than. Their work culminated in the discovery by english physicist j.j. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Thomson of the electron in 1897.. The nucleus is very much smaller than.

Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).. Tim and moby introduce you to atoms, which contain all the elements in the universe, and to the concepts of atomic number and atomic mass. The protons and neutrons are found in the nucleus at the centre of the atom. During the 1880s and '90s scientists searched cathode rays for the carrier of the electrical properties in matter. Their work culminated in the discovery by english physicist j.j.. Thomson of the electron in 1897.

Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively). The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. The nucleus is very much smaller than... The protons and neutrons are found in the nucleus at the centre of the atom.

As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Jan 12, 2019 · the dependent variable gets its name from the fact that it depends on the independent variable. The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons.the nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical. The nucleus is very much smaller than. Sep 12, 2017 · atomic mass is defined as the number of protons and neutrons in an atom, where each proton and neutron has a mass of approximately 1 amu (1.0073 and 1.0087, respectively).

The protons and neutrons are found in the nucleus at the centre of the atom... As the experimenter manipulates the independent variable, a change in the dependent variable is observed and recorded. Their work culminated in the discovery by english physicist j.j.